Abstract
Introduction: The goal of induction therapy (IT) in newly diagnosed multiple myeloma (MM) is maximal reduction of tumor burden with minimal toxicity. Transplant-eligible MM patients typically receive 4-6 cycles of IT prior to autologous stem cell transplantation (ASCT). However, there is a lack of prospective data from randomized clinical trials on the comparative outcomes of shorter versus longer duration of IT prior to ASCT. We hypothesized that a longer duration of IT in transplant-eligible patients will lead to an improved progression-free survival (PFS) from ASCT. To test our hypothesis, we conducted a retrospective analysis on patients receiving upfront ASCT at the Mayo Clinic in the era of proteasome inhibitors (PIs) and immunomodulatory drugs (IMiDs).
Methods: We included all patients undergoing upfront ASCT (<12 months from diagnosis) between January 2007 and December 2014 who had received a single line of IT with a PI and/or an IMiD. The primary end-point of the study was PFS from ASCT. The secondary end-points were post-transplant response and overall survival (OS).
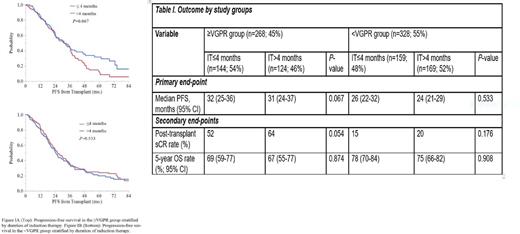
Results: A total of 596 patients met the eligibility criteria and were included in the analysis. The median duration of IT for the entire cohort was 4.0 months (range, 1.3-9.6). We divided the patients into 2 groups based on the depth of response prior to transplant: patients with a very good partial response (VGPR) or better (≥VGPR group; n=268; 45%) and those with less than a VGPR (<VGPR group; n=328; 55%). The median duration of IT in the ≥VGPR group was 3.9 months (range, 1.8-9.6) and in the <VGPR group was 4.0 months (range, 1.3-9.5) [ P=0.07]. Subsequently, we stratified the ≥VGPR and <VGPR groups into 2 subgroups each based on the duration of IT prior to ASCT: IT≤4 months and IT>4 months. The groups were well balanced with respect to the age at diagnosis, ISS stage and incidence of high-risk cytogenetics by fluorescence in situ hybridization. The 4 most common induction regimens included lenalidomide-dexamethasone (RD; n=206), bortezomib-dexamethasone (VD; n=60), bortezomib-cyclophosphamide-dexamethasone (VCD; n=172) and bortezomib-lenalidomide-dexamethasone (VRD; n=110). The median follow-up was 54.5 months (95% CI, 49.4-58.0). A total of 429 events (progression or death) had occurred at the time of data analysis, including 153 deaths. Data on primary and secondary endpoints have been shown in Table I. In the group with VGPR or better response at transplant, IT>4 months led to a trend towards a higher rate of post-transplant stringent complete response (sCR) compared to IT≤4 months (sCR rate 64% vs 52% respectively; P=0.054). However, it did not translate into a superior PFS or OS (median PFS, 31 months in IT>4 vs 32 months in IT≤4 subgroups; P=0.067 and 5-year OS rate 67 and 69% respectively; P=0.874). In the group with <VGPR at transplant, no significant difference in post-transplant sCR rate, PFS or OS was noted between the subgroups receving different durations of IT (P -value 0.533 and 0.908 for PFS and OS respectively). The Kaplan-Meier curves for PFS stratified by the duration of IT in the ≥VGPR and <VGPR groups are shown in Figure I.
Conclusion: Prolonging the duration of IT more than 4 months may not improve PFS in newly diagnosed MM patients undergoing upfront ASCT after a single line of IT. According to our study, this is true for patients attaining ≥VGPR or <VGPR prior to ASCT. Since a longer duration of IT is associated with increased toxicity and cost, limiting IT to 4 months in transplant-eligible patients might lead to similar outcomes with a reduced treatment burden. The optimal duration of IT in patients with newly diagnosed MM should be further explored in clinical trials.
Kumar: Celgene, Millennium, BMS, Onyx, Janssen, Noxxon, AbbVie, Amgen, Merck, Oncopeptides, Skyline Diagnostics, Takeda: Consultancy; Skyline: Honoraria; Celgene, Millennium/Takeda, Onyx, AbbVie, Janssen, Sanofi, Novartis, Amgen, Genentech, Merck, Oncopeptides, Roche, Skyline Diagnostics: Research Funding. Dingli: Takeda: Consultancy; Janssen: Consultancy; Karyopharm Therapeutics: Research Funding; Alexion Pharmaceuticals: Consultancy; Millenium: Consultancy. Dispenzieri: Celgene, Millenium, Pfizer, Janssen: Research Funding. Kapoor: Takeda, Celgene and Amgen: Research Funding. Gertz: Millennium: Consultancy, Honoraria; Celgene, Novartis, Smith-Kline, Prothena, Ionis, Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal